Electrochemical Biosensor Technology:
Quick, Accurate Self-Testing for the COVID-19 Antigen and Antibodies
Background
Over many years, Pepex has developed an extensive IP portfolio of over 300 global patents that protect our proprietary manufacturing process and unique biosensor design capable of detecting hundreds of unique biomarkers including COVID-19, Sepsis, HIV, Ebola, Hepatitis, and other life-altering antigens. The Pepex immuno-sensor can theoretically detect picomolar levels of antigen or antibodies in salvia or interstitial fluid such that the accuracy level of the test will easily exceed the current threshold of 95%. More specifically, our Lansing Sensor (LS™) technology is by design intended for use as an enzymatic or an immunologic probe, and based on recent worldwide events, Pepex is prepared to commercialize two COVID-19 products – one to detect the virus antigens (infections) and one to detect the virus antibodies (immunities) as described below.
COVID-19 Virus Antigen Detection
COVID-19 virus initially presents itself in saliva, mucous, and sputum. In order to detect COVID-19 virus antigen in saliva, we will use an antibody for the SARS-CoV-2 virus on our sensor. The test would be extremely sensitive and highly specific for only the intended virus. Once a sufficient sample volume is obtained, and without any further patient contact or interaction, the fluid collection is immediately analyzed using Pepex’s patented CCM® technology, and a quantitative readout is produced.
COVID-19 Virus Antibody Detection
To detect immunity (previously infected), the test paradigm is reversed. In this SARS-CoV-2 virus antigens on the sensor would detect for antibody presence in the patient. Being able to test for both Immunoglobulin M (IgM) and Immunoglobulin G (IgG) would allow further distinguishing recent versus remote infections, respectively. The COVID-19 antibody testing in interstitial fluid using Pepex’ patented Lansing Sensor (LS™) design automatically penetrates skin with a painless needle to a sufficient depth and then, as blood serum or interstitial fluid fills the test chamber, the needle retracks, removing contact with the patient. Without any further patient contact or interaction, the fluid sample is immediately analyzed using Pepex’s patented CCM® technology, and a quantitative readout is produced.
CCM® 3E™ LS™ AJP Manufacturing
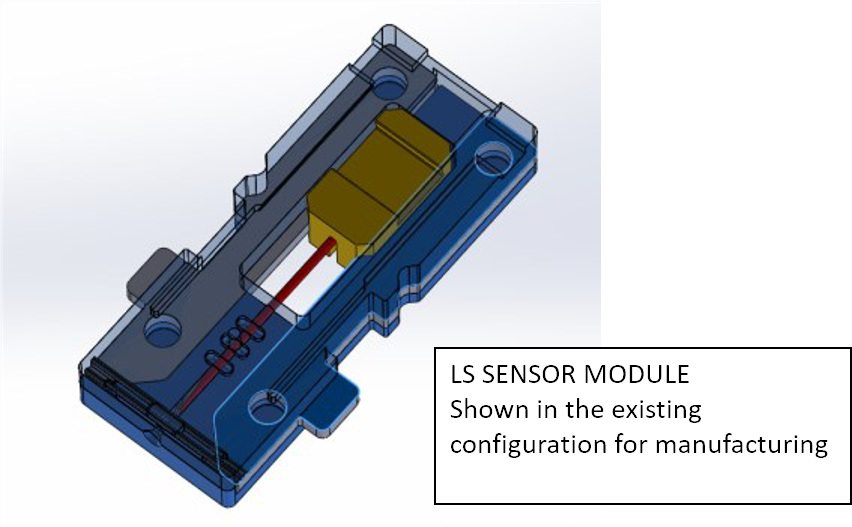
Pepex has developed significant proprietary advancements in manufacturing technique for the 3Electrode, Lancing Sensor, and Aerosol Jet Printing (AJP) platform. This unique production process provides significant enhancements to precision through the science of nanotechnology, adding meaningful benefits for high volume output while minimizing cost issues in all of our products. Prototype research has already been completed for both electrochemistry and engineering using the AJP techniques, but is redacted from this report on the advice of Patent Counsel.
Pilot Production
Refinements for final design will be concluded through precision automation on a Pilot Production System for final testing in humans using the CCM® 3E™ LS™ AJP Platform. An expedited approval process is expected via either EUA or 510(k) notification process along with demonstrable, repeatable manufacturing processes.
Prior FDA Collaboration
Concepts have been verified ex-vivo for 3E™ LS™, and the ‘general’ wired enzyme capability has been verified in YSI controlled animal models [both single and continuous monitors]. The matter of employing interstitial fluid for a single point system is mitigated by the experimentally defined testing times as currently accepted clinical decision-making standards. The presence of signal has been verified for LS™ 2E™ in vivo on hand-built sensors. The larger clinical application of interstitial fluid as a valid measure is defined by the other continuous monitors that are already FDA approved as a predicate device for Pepex FDA submission. This scenario simultaneously validates our 510(K) approach which will involve a relatively straight forward clinical trial requiring less scale [less than 500 patients] and a compressed timeline.
Electrochemical Performance of the 3E Needle Sensor
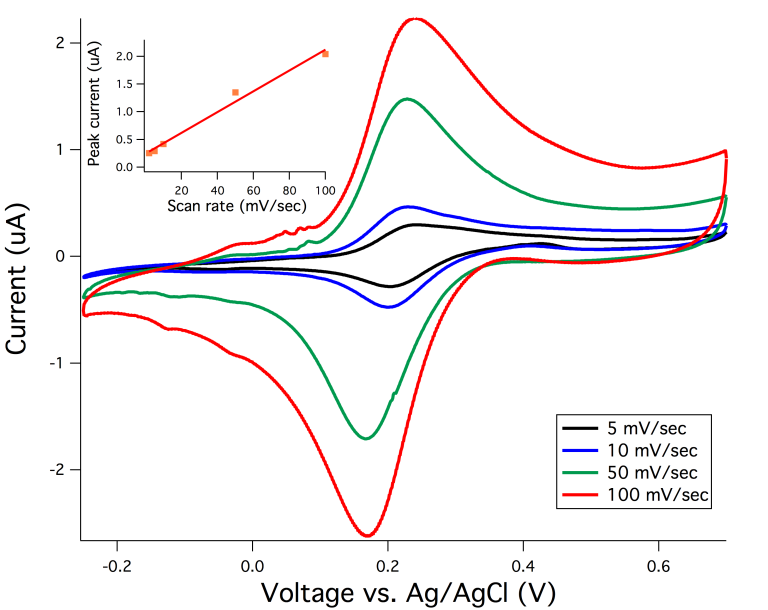
Pepex has previously assembled and validated electrochemical performance of the ultra-micro cell formed by the 3E needle sensors. Prior demonstrations prove that the 3E needle sensors are capable of operating as a high-quality electrochemical cell by measuring a reversible redox reaction of our mediator. Cycling voltammetry at increasing scan rates have been applied.
Representative cyclic voltammograms (CV) of each scan rate are shown in the graph, clearly demonstrating reversible electrochemical process with a clean CV signal. Continuous cycling at these scan rates indicated very stable and reproducible CV sweeps (data not shown). In summary, the 3E needle sensor is fully capable to provide the required electrochemical performance previously demonstrated with our mediator. The mediator undergoes anodic oxidation in the sensor and thus proves the electrochemical operational feasibility of the 3E needle sensor.
Features and Benefits
- Self Contained – eliminates requirement for any ancillary equipment or laboratory analysis.
- Ease of Use – no training required; may be administered personally or by healthcare providers.
- Highly Accurate – +95% for both antigens and antibodies; meets or exceeds commercial laboratory tests.
- Quick Results – positive test outcomes delivered in less than 15 minutes; negative test in 45 minutes.
- Sanitary – self-contained test module avoids biohazard disposal requirements.
- Low Unit Cost – disposable, fully contained sensor and module.
- Mass Production – high volume production processes are already defined and designed
Both designs essentially mimic either saliva sampling or blood draw and laboratory analysis, except that they are quicker, safer, and more convenient. Pepex’s new platform technologies are market-ready and combine novel design architecture, innovative chemistry, specialized chemistry coating methods, and unique manufacturing processes.
Current Requirements
Working closely in conjunction with our well-established industry partners Mikron and Insight, Pepex had developed a highly detailed three-month Project Plan to complete requisite laboratory refinement and initial production capability tasks.in preparation for FDA approval of two COVID-19 Lansing Sensor (LS®) systems, one for the detection of the COVID-19 virus antigen and the other for antibodies.
Abbreviations
CCM® – Conductive Composite Monofilament
- 2E™ LS™ – 2 Electrode Lansing Senor
- 3E™ LS™ – 3 Electrode Lansing Sensor
- AJP – Aerosol Jet Printing
- FDA – Food and Drug Administration
- EUA – Emergency Use Authorization
- 510(K) – Premarket submission to FDA demonstrating devices to be marketed are safe and effective
